
BE THE FIRST TO KNOW
Enter your email below to subscribe to the QLA newsletter to receive timely updates from your favorites products.
The basket mesh can become blocked for a number of reasons, including using the incorrect mesh size, blockage because of gummy excipients (other ingredients which make up the tablet), fast particulate release from the tablet, and air bubbles.
In all these cases the free flow of media through the basket can become impeded, and that can have a direct effect on the rate of dissolution. Observations should be made during the test to identify when and if these problems are occurring
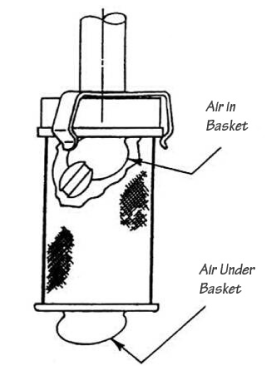 |
Air BubblesBubbles can be a problem with baskets. When a basket is lowered into the media, there is a danger that air bubbles can be trapped inside, or under the basket. The small hole in the basket hub is supposed to release air trapped inside but that does not always happen. (It is important to ensure that the hole in the basket hub remains clean and free from any blockage to enable air to escape). Media degassing is not the issue in this case, but a bubble trapped in or under the basket can easily invalidate the test. The dosage form in the basket can float to the bubble, thus preventing even dissolution. A bubble under the basket will prevent media movement through the mesh and therefore will have the same effect. Trapped air is a symptom of wet or dirty baskets being lowered into the medium, and if it does not dissipate immediately then the test will need to be aborted. Baskets manufactured from inferior materials or poorly made can suffer more from this problem than good baskets. |
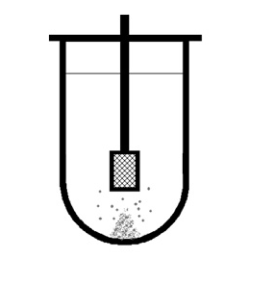 |
Low Media AgitationGiven a perfect basket, shaft, vessel and media, the basket test can be prone to generate remarkably little media movement, particularly at low RPM. With a disintegrating tablet, particles can clump together at the bottom of the media, an effect known as coning. There are numerous suggestions for avoiding this phenomenon from peak shaped vessels to eliminating the basket test altogether but none have been adopted by the USP thus far. However, for the foreseeable future this remains a potential issue, and any variations between vessel positions can have a marked effect. All the more reason to ensure that good baskets, vessels and shafts are used and that the physical validation and visual inspection of the system is carried out regularly. These areas will be covered in more detail later in this article. |
Due to the low media agitation, there is the potential for concentration gradients of active ingredient to be built up in the vessel. Correct and reproducible sample position then becomes important as well. This will be covered in more detail later.