
BE THE FIRST TO KNOW
Enter your email below to subscribe to the QLA newsletter to receive timely updates from your favorites products.
Selecting the ideal column for a given analysis depends largely on four factors. The polarity of the stationary phase, column length, internal diameter and film thickness.
The polarity of the stationary phase is chosen according to the compounds to analyze. Non polar phases, such as CGB-1 and CGB-5, separate compounds by their boiling points. Intermediate polarity phases such as CGB-WAX, CGB- 1701, combine boiling point with the more selective hydrogen bridge or dipolar moment interactions and thus provide a higher selectivity.
The polar phases such as TR-CN100 (Cyanosilicone with 100% of cyano propyl groups) use dipole-dipole interactions between the functional groups of the stationary phase and the analyte. These phases retain polar compounds more than non polar ones.
In general, non polar phases are more thermally stable than the polar phases. In other words, the higher the column polarity, the lower its thermal stability. Most of the Cronus columns are cross-linked, which results in high thermal stability. The cross-linking in a stationary phase produces slight changes in the physicochemical characteristics of the phase as well as in its polarity relative to the uncross-linked phase. Cronus also offers columns with non bonded phases that show the selectivity of the original phase (for instance CG-SE30, CG-SE54, CG-20M, etc.).
The resolving efficiency of a column (total number of theoretical plates) is a function of its length. The standard length used for most of the separations is 25-30 meters. This provides a high efficiency with relatively short analysis times. Columns of 15m are used for rapid control analyses, reaction monitoring, etc. as well as for the chromatography of high molecular weight substances while columns of 60m, 100m or 150m are used for very complex samples. Cronus manufactures a 150 m column for detailed analysis of petroleum and essential oil hydrocarbons.
As a general rule, for a constant temperature separation, the number of theoretical plates and analysis time are directly proportional to the column length while resolution is directly proportional to the square root of the theoretical plates (or length). Therefore if you double the column length, its resolution only increases by 40% whereas the analysis time doubles.
The internal diameter (ID) of a column is inversely proportional to its separation power. The smaller the diameter, the greater the efficiency (and thus a higher resolution) but at the same time the loading capacity decreases.
For samples containing a large number of substances where you may need a high resolution, small internal diameter columns (0.20-0.25 mm) are recommended. For samples with a high range of concentrations higher wider columns should be used (0.32-0.53 mm) since these larger diameters allow for the injection of a higher sample amount.
Columns of 0.53 mm ID (semicapillary) have a loading capacity similar to that of packed columns, but with better resolution, improved chemical inertness and lower analysis times.
The 0.32-0-53 mm ID columns can be used with either a standard injector (that is is used for capillary columns) or with a packed column injector, as they can operate at high flow rates. For GC-MS systems it is recommended to work with small ID columns (0.10mm, 0.15mm, 0.18mm, 0.20 mm and 0.22 mm) so as not to exceed the capacity of the MS vacuum system.
Capillary columns of 0.1 mm ID can generate high plate numbers and reduce analysis time without losing resolution. The high efficiency of these columns (7000-10000 plates/meter) allows the resolution of complex samples using shorter column lengths. However their loading capacity is a limiting factor and in order to obtain the best performance from these columns instrumental factors (injector/detector) have to be taken into account.
The film thickness of the stationary phase deposited inside the capillary column affects the efficiency, loading capacity, bleed level and the elution temperature of a compound.
A film thickness of 0.25-0.32 um is the standard thickness allowing for a compromise between loading capacity and resolution; and for the injection of samples with a wide volatility
range.
Thick films increase retention of the most volatile components whereas thin films provide faster elution at lower temperatures. As a general rule, thin films (0.1 um) must be used for compounds with a high molecular weight such as triglycerides, antioxidants etc., which have elution temperatures over 300°C. Thick films must be used for low boiling substances. Specifically, 3-5 μm films are used to separate solvents, gases, and very volatile substances at room temperature or lower.
When the thickness of the stationary phase increases, thermal stability decreases. Therefore the bleed level is higher and this can limit the maximum operating temperature of the column.
The β factor defines the relationship between the column ID and the stationary phase thickness. This helps to select the most appropriate column for your analysis.
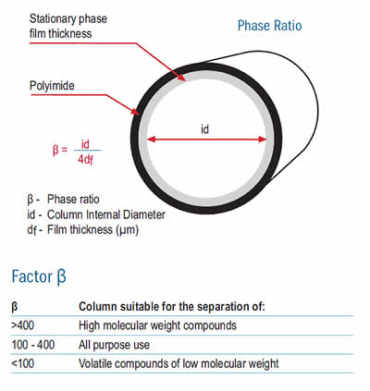
In addition, the β factor allows for easy method transfer since, for a given analysis with the same stationary phase, similar β factors will result in the same or very similar retention times and capacity factors. This assumes the column loading capacity (phase thickness and internal diameter) is acceptable.
The bleed level of stationary phase from a capillary column is directly related to the amount of stationary phase in the column and thus the film thickness. It also increases exponentially with temperature (fig.7). Excessive bleed can affect sensitivity and ultimately lower the limit of detection.
Cronus has a wide selection of capillary columns prepared with the most commonly used stationary phases (click here). Please contact us if you need help choosing a phase for your application.